Service hotline
+86 0755-83044319
release time:2022-03-17Author source:SlkorBrowse:10554
At present, there is still a gap between commercial high thermal conductivity Si3N4 ceramic substrate in China and foreign countries. Therefore, the research and development of Si3N4 ceramic substrate with high thermal conductivity will surely promote the great development of IGBT (Insulated Gate Bipolar Transistor) technology in China, and make a breakthrough in the high-end fields such as new energy.
In recent years, the actual thermal conductivity of silicon nitride ceramic substrate materials has been continuously improved, but there is still a big gap with the theoretical thermal conductivity. At present, the literature reports the methods to improve the thermal conductivity of silicon nitride ceramics, such as reducing the lattice oxygen content, promoting the crystal transformation, realizing the directional growth of grains, etc. This paper expounds how to improve the thermal conductivity of silicon nitride ceramics and realize the molding technology of mass production, focusing on the research progress of high thermal conductivity silicon nitride ceramics at home and abroad.
1 Effect of lattice oxygen
The main heat transfer mechanism of silicon nitride is lattice vibration, which conducts heat through phonons. The vibration of lattice is not linear, and there is a certain coupling effect between lattices. Phonons will collide with each other, which will reduce the average free path of phonons. In addition, all kinds of defects, impurities and grain interfaces in Si3N4 crystal will cause phonon scattering, which is equivalent to the reduction of phonon mean free path, thus reducing thermal conductivity. Fig. 3 shows the microstructure of silicon nitride.
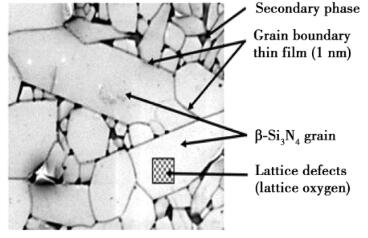
Fig. 3 Typical microstructure of silicon nitride sintered body
The results show that lattice oxygen is one of the main defects affecting the thermal conductivity of silicon nitride ceramics among many lattice defects. In the process of sintering, the following solid-solution reaction will take place in oxygen:
2SiO2→ 2SiSi +4ON+VSi (1)
In the reaction, silicon vacancies are generated, and atomic substitution will cause some distortion of the crystal, which will cause phonon scattering, thus reducing the thermal conductivity of Si3N4 crystal.
Kitayama et al. systematically studied the factors affecting the thermal conductivity of Si3N4 crystal from two aspects: lattice oxygen and grain boundary phase, and found that the size of Si3N4 crystal will change the degree of influence of the above factors. When the grain size is less than 1μm, the thickness of lattice oxygen and grain boundary phase will become the main factors affecting the thermal conductivity. When the grain size is larger than 1μm, lattice oxygen is the main factor affecting the thermal conductivity. However, the preparation of silicon nitride ceramics with high thermal conductivity requires large crystal grains. Therefore, it is particularly critical to prepare silicon nitride with high thermal conductivity by reducing the content of lattice oxygen. The effective methods to reduce the content of lattice oxygen are described from the following aspects: the selection of raw materials, the selection of sintering AIDS and the reduction of carbon in the preparation process.
1.1Raw material selection
In order to reduce the oxygen content in silicon nitride crystal lattice, it is necessary to reduce the impurity oxygen content from the raw powder. At present, there are two methods: one is to use Si powder with low oxygen content as raw material, and obtain Si3N4 ceramics with high density and high thermal conductivity through two steps of nitriding and re-sintering of Si powder. The dense body of Si composed of Si powder and sintering aid is heated to the temperature near the melting point of Si (1414℃) in nitrogen atmosphere, so that Si is nitrided and transformed into porous Si3N4 sintered body, which is further heated to a higher temperature to sinter porous Si3N4 into dense Si3N4 ceramics. The other is sintering with high purity α-Si3N4 powder with lower oxygen content, or directly sintering with β-Si3N4.
Zhou and Zhu of Japan have prepared a series of silicon nitride ceramics with thermal conductivity over 150 W/(m k) by SRBSN process with Si powder as raw material. The main reason for the high thermal conductivity is that compared with ordinary commercial α-Si3N4 powder, Si powder after nitriding has less oxygen content and impurities.
Park et al. studied the influence of the particle size of raw material Si powder on the thermal conductivity of silicon nitride ceramics, and found that the reduction of the particle size of Si can narrow the silicon nitride channel, which is conducive to the elimination of pores in the sintering process, thus obtaining high density silicon nitride ceramics. The results show that when the Si powder is reduced to 1μm, the relative density of silicon nitride ceramics can reach over 98%. However, in the process of reducing the particle size of raw materials by SRBSN, the surface of raw materials is easily oxidized, and the content of lattice oxygen in raw materials is increased.
Guo et al. made a comparative experiment with Si powder and α-Si3N4 as raw materials. It is found that Si3N4 powder with low oxygen content (0.36%, mass fraction) can be obtained by nitriding Si powder, and the silicon nitride ceramic with thermal conductivity of 66.5 W/(m k) can be prepared by pressureless sintering. Under the same conditions, the thermal conductivity of silicon nitride ceramics made of α-Si3N4 is only 56.8 W/(m k).
Silicon nitride ceramics with high thermal conductivity can also be prepared by using high purity α-Si3N4 powder as raw material. Duan et al. prepared α-Si3N4 ceramics with density, thermal conductivity, bending strength, fracture toughness and Vickers hardness of 3.20g cm-3, 60w/(m k), 668 MPa, 5.13mpa m1/2 and 15.06 GPa, respectively. Kim et al. prepared silicon nitride ceramics with thermal conductivity of 78.8 W/(m k) from α-Si3N4.
Liu Xingli et al. prepared silicon nitride ceramics with thermal conductivity of 108 W/(m k) and bending strength of 626 MPa by using different proportions of β-Si3N4/α-Si3N4 powder as starting materials. The results show that with the increase of β-Si3N4 powder content, the average length-diameter ratio of β-Si3N4 columnar grains decreases, resulting in the decrease of grain bulk density, the corresponding increase of columnar crystal integral number, the decrease of intergranular phase content and the increase of thermal conductivity.
Peng Mengmeng et al. studied the influence of powder type (β-Si3N4 or α-Si3N4) and SPS holding time on the thermal conductivity of silicon nitride ceramics. It is found that the thermal conductivity of silicon nitride ceramics prepared by β-Si3N4 powder is 15% higher than that of silicon nitride ceramics prepared by α-Si3N4 powder by the same process, reaching 105 W/(m k). See Table 1 for comparison of thermal conductivity of Si3N4 materials prepared from different raw materials.
Table 1 Comparison of thermal conductivity of Si3N4 materials prepared from different raw materials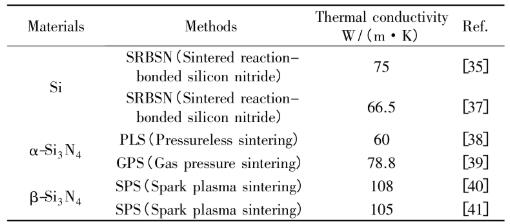
According to the above comprehensive research, the samples made of Si powder can achieve high thermal conductivity, but it is easy to be oxidized in the grinding process, and the experimental process is tedious and takes a long time, which is not conducive to industrial production. When α-Si3N4 powder with high purity and low oxygen content is used as raw material, silicon nitride ceramics with excellent performance can be prepared because of its high purity, but this will lead to the increase of cost, which is not conducive to large-scale production. Although β-Si3N4 can be used instead of α-Si3N4 as raw material to obtain silicon nitride ceramics with high thermal conductivity, the rod-shaped grains of β-Si3N4 will hinder grain rearrangement, resulting in difficult densification of sintered products.
1.2 Selection of sintering AIDS
Si3N4 is a covalent compound with a small self-diffusion coefficient, and it is difficult to form a dense crystal structure by self-diffusion during sintering. Therefore, silicon nitride ceramics with high thermal conductivity can be obtained by adding appropriate sintering AIDS and optimizing the ratio of sintering AIDS. At high temperature, sintering aid reacts with SiO2 on Si3N4 surface to form liquid phase.Finally, the grain boundary phase is formed. However, the thermal conductivity of grain boundary phases is only 0.7 ~ 1 W/(m k), which greatly reduces the thermal conductivity of silicon nitride. Moreover, the introduction of some oxide sintering additives will lead to the increase of lattice oxygen content of Si3N4 and the decrease of thermal conductivity.
At present, there are many kinds of sintering AIDS for silicon nitride ceramics, including various rare earth oxides, magnesium compounds, fluoride and their composite sintering AIDS. Rare earth elements are often used to adsorb oxygen from Si3N4 lattice because of their high oxygen affinity. At present, the commonly used mixed sintering aids are magnesium oxide and rare earth oxide.
Jia et al. added Y2O3-MgO in the sintering process of silicon nitride ceramics to prepare silicon nitride ceramics with thermal conductivity of 64.4 W/(m k). Go also used Y2O3-MgO as sintering aid, and studied the influence of the particle size of sintering aid MgO on the microstructure and thermal conductivity of silicon nitride. It is found that the addition of coarse MgO particles will lead to the uneven distribution of liquid phase composition during sintering, which will lead to the preferential growth of Si3N4 grains around the MgO-rich region, thus leading to the increase of the proportion of large Si3N4 grains in the final Si3N4 ceramics and the improvement of thermal conductivity.
However, adding oxide sintering AIDS will inevitably introduce oxygen atoms, so in order to reduce the oxygen impurities in the crystal lattice, oxide+non-oxide can be used as sintering AIDS. Yang et al. prepared high thermal conductivity silicon nitride ceramics with MgF2-Y2O3 as sintering additive. It was found that MgF2 can reduce the viscosity of liquid phase, accelerate particle rearrangement, and make the powder mixture densify at a lower temperature (1600℃) and a shorter time (3 min). Moreover, low liquid phase viscosity and high Si/N atomic ratio contribute to the α→β phase transition and grain growth of Si3N4 ceramics, thus improving the thermal conductivity of Si3N4 ceramics.
Hu, et al. conducted a comparative test with MgF2-Y2O3 and MgO-Y2O3 as sintering AIDS, and explored the influence of the ratio of sintering AIDS on thermal conductivity. Compared with MgO-Y2O3, the thermal conductivity of Si3N4 ceramics is increased by 19% when MgF2-Y2O3 is used as sintering aid, and the highest thermal conductivity can be achieved when the addition amount is 4%MgF2 -5%Y2O3.
Li used Y2Si4N6C-MgO instead of Y2O3 -MgO as sintering additive. By introducing nitrogen and promoting the elimination of silica, a high nitrogen-oxygen ratio was formed in the second phase, which resulted in the increase of grain size, the decrease of lattice oxygen content, the increase of continuity of Si3N4 -Si3N4, and the increase of thermal conductivity of Si3N4 ceramics from 92 W/(m k) to 120 W/(m k).
In order to further improve the nitrogen-oxygen ratio in the liquid phase and reduce the lattice oxygen content, non-oxides are usually used as sintering AIDS. The effects of oxide and non-oxide sintering additives on the microstructure, thermal conductivity and mechanical properties of Si3N4 were studied by Lee. With MgSiN2 -YbF3 as sintering additive, Si3N4 ceramic material with thermal conductivity of 101.5 w/(m k) and bending strength of 822~916 MPa was prepared. Compared with oxide sintering additives, non-oxide MgSiN2 and fluoride as sintering additives can reduce the secondary phase and lattice oxygen content of silicon nitride, in which rare earth fluoride can react with SiO2 to generate SiF4, and the evaporation of SiF4 leads to the reduction of grain boundary phase and the reduction of grain boundary phase SiO2, thus achieving the purpose of improving thermal conductivity. The comparison of thermal conductivity of silicon nitride ceramics prepared with different sintering AIDS is shown in Table 2, and the microstructure is shown in Figure 4.
Table 2 Comparison of thermal conductivity of Si3N4 materials prepared with different sintering AIDS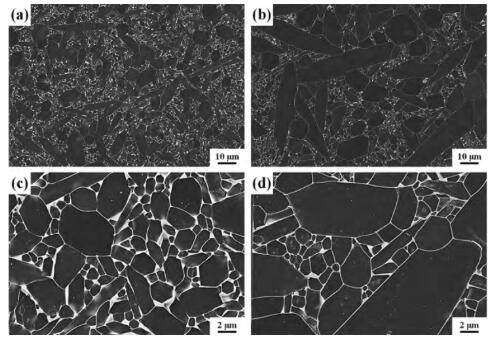
Fig. 4 Microstructure of oxide additives (a)MgO-Y2O3 and (d)MgO-Yb2O3, mixed additives (b)MgSiN2 -Y2O3 and (e)MgSiN2 -Yb2O3, non-oxide additives (c)MgSiN2 -YF3 and (f) Mg-Sin2-YF3.
At present, the rare earth elements in the mainstream sintering AIDS are compounds of Y and Yb, but some rare earth elements can't improve the density. Guo et al. respectively used ZrO2 -MgO-Y2O3 and Eu2O3 -MgO-Y2O3 as sintering AIDS to prepare silicon nitride ceramics. It was found that the addition of Eu2O3 -MgO-Y2O3 inhibited the densification of silicon nitride ceramics.
According to the above research, compared with oxide sintering aids, non-oxide sintering aids can provide additional nitrogen atoms, increase the ratio of nitrogen to oxygen, promote crystal transformation, and reduce SiO2 to reduce the content of lattice oxygen and grain boundary phase.
1.3 Reduction of carbon
The aforementioned sintering AIDS, such as Y2S4N6C and MgSiN2, which can effectively reduce the content of lattice oxygen, cannot be obtained from commercial sources, thus causing troubles for large-scale production, and high-temperature heat treatment will also lead to high cost. Therefore, from the perspective of industrial application, it is of great significance to develop a simple and cheap preparation method of high thermal conductivity Si3N4 ceramics. It is found that doping a certain amount of carbon in the sintering process can reduce oxygen impurities, which is an effective method to reduce the lattice oxygen content.
Carbon is widely used as a sintering additive for non-oxide ceramics, and its main function is to remove oxide impurities from the surface of non-oxide powder. On this basis, the researchers found that the addition of a small amount of carbon can effectively reduce the lattice oxygen content of AlN ceramics, thus improving the thermal conductivity of AlN ceramics. Similarly, the introduction of carbon into Si3N4 ceramics can also reduce the oxygen content, mainly because in the process of nitridation and post-sintering, an appropriate amount of carbon will play a very obvious reduction role, which can greatly reduce the partial pressure of SiO and increase the N/O atomic ratio of the intergranular secondary phase, thus forming a bimodal microstructure, obtaining large and slender silicon nitride particles, and improving the thermal conductivity of silicon nitride ceramics.
Li When BN/ graphite is used instead of BN as powder base plate, the thermal conductivity of silicon nitride ceramics is increased by 40.7%. It is found that the thermal conductivity of silicon nitride ceramics can still reach 121 W/(m k) even if the oxygen content of Si powder reaches 4.22% after ball milling. The main reason is that graphite has strong reducing ability. In the process of nitriding, it can promote the removal of SiO2, change the chemical composition of the secondary phase, and further promote the elimination of SiO2 and Y2Si3O3N4 secondary phases in the process of sintering, thus making the product produce larger rod-like grains, reducing the lattice oxygen content and improving the continuity of Si3N4 -Si3N4. The results show that although some carbon is doped, the resistivity of silicon nitride remains unchanged, but the final product has a high mass loss ratio (25.8%), which increases the cost of raw material loss.
Li et al. found that excessive graphite will react with Si3N4 on the surface, which is the key factor leading to high mass loss ratio of silicon nitride ceramics. So they improved the preparation process, adopted the two-step gas pressure sintering method, and used the powder mixture of 5% (mole fraction) carbon doped with 93%α-Si3N4 -2%Yb2O3 -5%MgO as raw material for sintering experiment. The results show that the thermal conductivity of Si3N4 ceramics is increased from 102 W/(m k) to 128 W/(m k) by 25.5% with the addition of carbon. In the first sintering process, the carbothermal reduction process significantly reduced the oxygen content and increased the N/O ratio of the intergranular secondary phase. In the semi-finished Si3N4 samples, Y2Si4O7N2 secondary phase appeared, the content of β-Si3N4 was higher, and the rod-shaped β-Si3N4 grains were larger. In the second sintering process, the second phase Y2Si4O7N2 reacts with carbon to form YbSi3N5, which greatly reduces the lattice oxygen content, resulting in coarser rod-shaped grains and tighter Si3N4 -Si3N4 interface, which significantly improves the thermal conductivity of Si3N4 ceramics. The SEM image of the prepared Si3N4 is shown in Figure 5.

Fig. 5 SEM micrographs of the polished surface and plasma etched surface of the final Si3N4 ceramic sample: (a) low-power images of a)SN and (b)SNC; High power images of (c)SN and (d)SNC
Adding carbon into silicon nitride ceramics with high thermal conductivity is an effective method to reduce lattice oxygen content. This method has low requirements on oxygen content of raw materials and sintering AIDS, which reduces the preparation cost of silicon nitride ceramics with high thermal conductivity. With the continuous improvement of technology, it is expected to be applied in industrial production.
2 crystal transformation and crystal axis orientation.
2.1 Influence of crystal transformation on thermal conductivity and improvement methods
The thermal conductivity of β-Si3N4 is higher than that of α-Si3N4 because of its more symmetrical structure. In the process of sintering silicon nitride ceramics at high temperature, the low-temperature phase α-Si3N4 of the raw material will be transformed into the high-temperature phase β-Si3N4 through the dissolution-precipitation mechanism, but the crystal type transformation is not complete in the sintering process, and the untransformed α-Si3N4 will greatly affect the thermal conductivity of silicon nitride ceramics. In order to promote the crystal transformation and obtain a higher β/(α+β) ratio, the commonly used methods at present are: (1) changing the sintering system, such as increasing the sintering temperature, prolonging the sintering time and subsequent heat treatment; (2) A proper amount of β-Si3N4 rod-shaped crystal grains are added into α-Si3N4 as seed crystals. Fig. 6 shows the bimodal microstructure distribution of silicon nitride ceramics after seed addition.
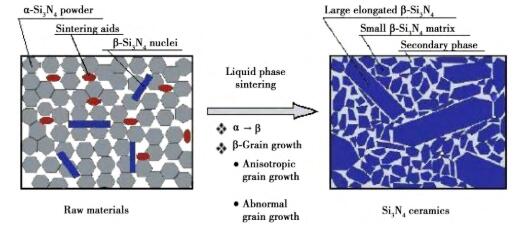
Fig. 6 Dual-mode microstructure distribution of β-Si3N4 ceramics after adding seed crystal.
Zhou et al. explored the effects of different sintering time on thermal conductivity, bending strength and fracture toughness of silicon nitride ceramics. It can be seen from Table 3 that the thermal conductivity of silicon nitride ceramics gradually increases with the prolonging of sintering time. This is mainly due to the continuous growth of crystal grains, the increasing of β-Si3N4 content and the decreasing of lattice oxygen content with the process of dissolution and precipitation. Tong Wenxin et al. studied the effect of sintering temperature on the thermal conductivity of Si3N4, and found that the samples sintered at 1600℃ contained both α phase and β phase. After the sintering temperature rises to 1700℃ and 1800℃, only β phase exists in the sample. With the increase of sintering temperature, the thermal conductivity of the sample increases, which may be caused by the increase of grain size, the decrease of liquid content and the formation of independent "glass capsule" in liquid phase at the edge of multiple grain boundaries.
Table 3 Performance comparison of Si3N4 at different sintering time
Zhu et al. found that adding β-Si3N4 as seed crystal during sintering can obtain silicon nitride ceramics with higher densification degree and thermal conductivity. In order to further promote the crystal transformation and obtain large-sized silicon nitride grains, β-Si3N4 can be used instead of α-Si3N4 as starting powder to prepare high thermal conductivity silicon nitride ceramics. Liang Zhenhua, etc. added 1% (mass fraction) rod-shaped β-Si3N4 particles as seed crystal, and the thermal conductivity of silicon nitride ceramics reached 158 W/(m k). Liu Xingli et al. explored the influence of different proportions of β-Si3N4/α-Si3N4 on the thermal conductivity and mechanical properties of silicon nitride ceramics. The results showed that when all the raw materials were β-Si3N4, the thermal conductivity of silicon nitride ceramics was the highest, reaching 108 W/(m k), but the bending strength also decreased.
According to the above research, increasing sintering temperature and prolonging sintering time can promote crystal transformation to a certain extent. Adding a proper amount of β-Si3N4 seed crystal to promote the crystal transformation can increase the β/(α+β) ratio in a short time, make the crystal grain grow more fully, and obtain silicon nitride ceramics with high thermal conductivity.
2.2 Influence of crystal orientation on thermal conductivity and its improvement method
Because the growth rate of the C axis is higher than that of the A axis, the anisotropic growth leads to the rod shape of β-Si3N4 and the anisotropy of its physical properties. The thermal conductivity of silicon nitride grains is anisotropic. The theoretical thermal conductivities of β-Si3N4 single crystal along the A axis and the C axis are 170 W/(m k) and 450 W/(m k) respectively. Therefore, proper methods can be adopted in the molding process to realize the directional arrangement and promote the directional growth of silicon nitride grains. At present, the molding methods that can make grains grow directionally include tape casting molding, hot pressing molding and grouting molding.
Under the action of external magnetic field, silicon nitride crystal has obvious growth difference along each crystal axis. This is mainly due to the difference of magnetic susceptibility of silicon nitride crystal along each crystal axis. Under the action of external strengthening magnetic field, silicon nitride crystal will be subjected to the action of torque. By rotating a certain angle to have the minimum magnetization energy, the expression of silicon nitride crystal rotation driving energy is as follows:
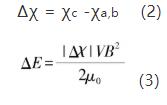
Where: V is the volume of particles, B is the applied magnetic field, μ0 is the magnetic permeability in vacuum, χc and χa, B represent the magnetic susceptibility of silicon nitride crystal along the C axis and A, B axis, respectively, and | Δ χ| is the absolute value of the magnetic susceptibility difference of the crystal along each crystal axis. And the expression of thermal motion energy u of particles is:
U=3nN0kB (4)
Where: n is the number of moles of material particles, N0 is Avogadro's constant, kB is Boltzmann's constant, and t is temperature. When δ e is greater than u, particles can be rotated by magnetic field. As can be seen from fig. 7, if the c axisWith high magnetic susceptibility, rod-shaped particles will be arranged parallel to the magnetic field; If the magnetic susceptibility of the C axis is low, the rod-shaped particles will be aligned perpendicular to the magnetic field.
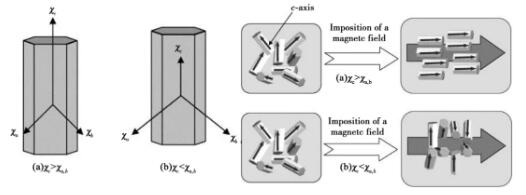
Fig. 7 Schematic diagram of the influence of magnetic field on the arrangement of hexagonal rod-shaped particles in the lattice: (a) χ c > χ a, b; (b) χc<χa,b
Samples with oriented microstructure can be prepared by introducing strong magnetic field in the forming process of weak magnetic ceramics. As the magnetic susceptibility χ c < χ a, b of silicon nitride grains along each axis, silicon nitride ceramics with C-axis orientation can be prepared by grouting and other techniques in a rotating horizontal magnetic field. The preparation principle is shown in Figure 8.
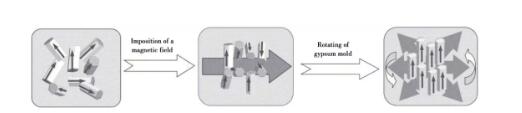
Fig. 8 Preparation of ceramics with crystal axis orientation in magnetic field
Yang Zhigang et al. used gel injection molding instead of traditional grouting molding to prepare textured silicon nitride ceramics oriented along axis A or B in 6T longitudinal magnetic field, and studied the effects of sintering temperature and holding time on the texture of silicon nitride ceramics. The results show that increasing sintering temperature promotes the texture of silicon nitride ceramics, while prolonging sintering time has little effect on the texture. Liang et al. found that the silicon nitride crystal grain {0001} showed signs of growth along the Z axis and had strong orientation when preparing silicon nitride ceramics by hot pressing sintering. This is beneficial to the preparation of silicon nitride ceramics with high thermal conductivity. Zhu et al. performed grouting molding in a horizontal magnetic field of 12T, and obtained high thermal conductivity silicon nitride ceramics with a thermal conductivity of 170w/(m k). It is found that during the grouting process, the mold rotates at a speed of 5 r/min to form a rotating magnetic field, which leads to the C-axis orientation of β-Si3N4 perpendicular to the magnetic field during the solidification process, and the C-axis orientation coefficient is 0.98. Fig. 9 shows the influence of magnetic field and die rotation on the grain orientation of rod-shaped silicon nitride.

Fig. 9 Effect of magnetic field and die rotation on grain orientation of rod-shaped silicon nitride
At present, it is difficult to realize the oriented growth of silicon nitride grains in large-scale production. At present, the oriented growth of silicon nitride ceramics reported in the literature is limited to the laboratory stage, and it is necessary to realize the oriented growth of silicon nitride grains in industrial production through appropriate methods, which has great application prospects for preparing high thermal conductivity silicon nitride ceramics.
3 Ceramic substrate preparation process
3.1 moulding process
Due to the miniaturization of power electronic devices, the size and thickness of silicon nitride ceramic substrates are required to be more precise. The thickness range of commercial silicon nitride ceramic substrates is 0.3~0.6 mm In order to realize large-scale production of silicon nitride ceramic substrate materials, it is particularly important to choose an appropriate molding method. At present, there are many molding methods to prepare silicon nitride ceramics, such as tape casting, hot pressing, grouting, cold isostatic pressing, etc. However, in order to meet the requirements of miniaturization and refinement and realize the directional growth of silicon nitride grains at the same time, tape casting is undoubtedly the key to achieve this goal. Fig. 10 is a flow chart of the casting process, and the preparation of silicon nitride ceramic substrate materials by casting is described below.
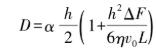
Fig. 10 Flow chart of tape casting process
The slurry of tape casting is the most critical factor to determine the performance of green body. The slurry includes powder, solvent, dispersant, binder, plasticizer and other additives. Each component has an important influence on the performance of the slurry, and each component in the slurry will also influence each other. Although tape casting has unique advantages compared with other molding processes, in practice, due to different stress release mechanisms, it is easy to cause bending, cracking, wrinkling, uneven thickness and other phenomena when the tape casting sheet is dried. In order to prepare uniform and stable casting slurry and smooth casting sheet after drying, it is necessary to pay attention to factors such as slurry wettability, stability and blank thickness while keeping the formula unchanged.
When silicon nitride casting sheet is prepared by casting, Otsuka et al. and Chou et al. put forward the theoretical liquid flow model, and the relationship between casting sheet thickness D and casting parameters is shown in formula (5):
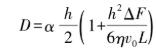 (5)
(5)
Where: α represents the shrinkage coefficient of the thickness of the wet blank when it is dried, and the viscosity and uniformity of the slurry have great influence on it; H and l represent the height and length of the blade gap of the scraper respectively; η represents the viscosity of the slurry; F represents the pressure in the hopper, which is generally determined by the height of the slurry; V represents the relative speed of the casting device and the support carrier. In order to prepare the ultra-thin ceramic substrate, it is necessary to properly adjust the blade gap and keep the liquid level of the slurry constant while keeping the viscosity and uniformity of the slurry moderate.
In organic tape casting, generally, azeotrope is used as solvent, and the dissolution effect is better, so it is necessary to ensure that the solvent has good wettability to powder particles, which is related to the surface tension of the solvent, which can be explained by formula (6):
 (6)
(6)
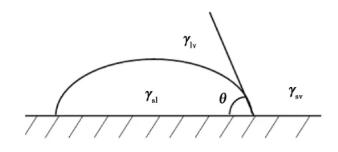
Where θ is the wetting angle; γsv, γsl and γlv represent the surface tension of solid-gas, solid-liquid and liquid-gas respectively. It can be seen from formula (6) that the smaller γlv is, the smaller θ is, indicating the better wettability. The wetting action is shown in Figure 11.

Fig. 11 schematic diagram of wetting action
In order to ensure the uniformity and stability of casting slurry, it is necessary to add dispersant, whose main function is to make the surface of powder particles easy to wet, reduce the surface potential energy of powder particles to make them easier to disperse, and raise the potential barrier between particles, thus making the slurry stable and uniform. The stability of slurry can be described by DLVO theory:
UT=UA+UR(7)
Where: UA is Van der Waals gravitational potential energy; UR is repulsive potential energy. When UR is greater than UA, the slurry is stable. In order to ensure the uniformity and stability of slurry, the dosage of dispersant should also be controlled. If the dosage is too much, the produced particles are easy to bond, which is not conducive to obtaining beaded particles; If the dosage is too small, it is easy to be dispersed into small droplets, and the monomer is unstable. With the progress of the reaction, the dispersed droplets may also condense into blocks. Duan et al. first prepared a casting sheet with uniform microstructure and a relative density of 56.08% by casting process, and then obtained a silicon nitride ceramic with a relative density of 99% and a thermal conductivity of 58 W/(m k) by gas pressure sintering. Zhang et al. prepared dense silicon nitride ceramics with thermal conductivity of 81 W/(m k) by tape casting process and gas pressure sintering process. It is found that the highest thermal conductivity can be obtained when dispersant (PE), binder (PVB), plasticizer/binder ratio and solid load are 1.8% (mass fraction), 8% (mass fraction), 1.2% and 33% (volume fraction) respectively. Zhang Jingxian, et al. first prepared the cast sheet of Si by tape casting, and then prepared the silicon nitride ceramic with thermal conductivity of 76 W/(m k) by degreasing, nitriding and sintering. At present, the thermal conductivity of silicon nitride ceramics prepared by tape casting is not high, far lower than the level reported in the literature (> 150 W/(m k)). It is the key to prepare uniform and stable slurry with moderate viscosity and good wettability by improving the process and optimizing the ratio of each component.
3.2 Sintering process
At present, the main sintering methods for preparing silicon nitride ceramics are gas pressure sintering, reaction sintering, re-sintering, spark plasma sintering, hot pressing sintering, etc. Each method has its own advantages and disadvantages. Here are some common sintering methods briefly summarized.
Gas pressure sintering (GPS) can rapidly densify silicon nitride in nitrogen atmosphere by pressurizing and heating, promote the rapid transformation of α→β crystal form, and help to improve the thermal conductivity of silicon nitride ceramics. Li, etc. Silicon nitride ceramics with high thermal conductivity were prepared by two-step gas pressure sintering method with α-Si3N4 as raw material. Firstly, the mixed powder was heated to 1500℃ under the nitrogen pressure of 1 MPa and then sintered at 1900℃ for 12h. Through two-step gas pressure sintering reaction, the crystal transformation of α→β-Si3N4 was greatly promoted, and the thermal conductivity of silicon nitride ceramics reached 128W/(m k). Kim et al. heated to 1900℃ in nitrogen atmosphere of 0.9 MPa by gas pressure sintering method, and kept the temperature for 6 hours. Finally, the thermal conductivity of silicon nitride ceramics was 78.8 W/(m k). Li et al. Silicon nitride ceramics with thermal conductivity of 120 W/(m k) were prepared by gas pressure sintering method with Y2Si4N6C-MgO as sintering aid.
Plasma sintering (SPS) process is a kind of sample sintering method which realizes the joint action of pressure field, temperature field and electric field. It has the advantages of fast heating rate, low sintering temperature and short sintering time. Yang et al. prepared Si3N4 ceramics with thermal conductivity of 76 w/(m k), bending strength of 857.6 MPa, hardness of 14.9 GPa and fracture toughness of 7.7 MPa m 1/2 by SPS process with MgF2-Y2O3 as sintering additive. The experiment shows that due to the action of the applied electric field, the particles are easy to slide, which is conducive to the rearrangement of particles, thus obtaining large-grained particles, and making Si3N4 achieve higher densification at a lower temperature.
Hu et al. prepared Si3N4 ceramic materials with thermal conductivity of 82.5 w/(m k), bending strength of (911±47) MPa and fracture toughness of (8.47±0.31) MPa·m1/2/2 by SPS process with MgF2-Y2O3 and MgO-Y2O3 as sintering additives. SPS process can also solve the above-mentioned problem that it is difficult to sinter silicon nitride ceramics with β-Si3N4 as raw material. Peng Mengmeng et al. sintered at 1600℃ for 5 min by SPS process, then kept at 1900℃ for 3h, and obtained dense silicon nitride ceramics with thermal conductivity as high as 105 W/(m k). Liu et al. successfully prepared high thermal conductivity silicon nitride ceramics with density up to 99% by SPS and heat treatment process with different proportions of β-Si3N4 /α-Si3N4 powder as starting materials.
Reactive re-sintering (SRBSN) is favored by researchers because the porous Si3N4 sintered body is obtained by nitriding Si powder, and then sintered to form dense silicon nitride ceramics, which has lower oxygen content than the silicon nitride ceramics prepared from commercial α-Si3N4. Zhou et al. prepared Si3N4 ceramics with thermal conductivity as high as 177 W/(m k) by SRBSN process. The results show that the higher thermal conductivity can be obtained by prolonging the sintering time and further reducing the lattice oxygen content. In addition, they also studied the fracture behavior of Si3N4 ceramics with high thermal conductivity, and found that it had a high fracture toughness (11.2 MPa m1/2). Zhou et al. prepared Si3N4 ceramics by SRBSN process with Y2O3 and MgO as additives. It is found that the content of Y2O3 -MgO additive and sintering time will affect the thermal conductivity of Si3N4. When the additive content is 2%Y2O3 -4%MgO, the thermal conductivity of Si3N4 ceramics is 156 w/(m k) after sintering for 24 h, which is 21% higher than that of Si3N4 ceramics (128 w/(m k)) after sintering for 6 h. Li Si3N4 ceramics with thermal conductivity as high as 121 W/(m k) were prepared by SRBSN process with Y2O3-MgO as sintering aid.
Silicon nitride ceramics with high thermal conductivity can also be prepared by other sintering methods. Jia et al. prepared silicon nitride ceramics with thermal conductivity of 64.6 W/(m k) by ultra-high pressure sintering. Duan et al. prepared silicon nitride ceramics with thermal conductivity of 60 W/(m k) by low temperature pressureless sintering at 1780℃ with 10% TiO2 -MgO as sintering additive. Lee et al. prepared silicon nitride ceramics with thermal conductivity of 101.5 W/(m k) by hot pressing sintering process.
According to the above research, although the sintering methods are different, silicon nitride ceramics with excellent properties can be prepared. In order to realize the large-scale production of silicon nitride ceramics, factors such as cost, operation difficulty and production cycle need to be considered, so it is the key to find a fast, simple and low-cost sintering process.
4tag
Si3N4 ceramic is more and more popular in the field of high-power semiconductor devices because of its potential high thermal conductivity and excellent mechanical properties, and it is expected to become the preferred ceramic substrate material for electronic devices. However, there are many factors that limit its thermal conductivity, such as lattice defects, impurity elements, lattice oxygen content, grain size, etc., which cause the actual thermal conductivity of silicon nitride ceramics to be low.
Disclaimer: This article is reproduced from "5G Industry Watch". This article only represents the author's personal views, not the views of Sacco Micro and the industry. It is only for reprinting and sharing to support the protection of intellectual property rights. Please indicate the original source and author when reprinting. If there is any infringement, please contact us to delete it.



Site Map | 萨科微 | 金航标 | Slkor | Kinghelm
RU | FR | DE | IT | ES | PT | JA | KO | AR | TR | TH | MS | VI | MG | FA | ZH-TW | HR | BG | SD| GD | SN | SM | PS | LB | KY | KU | HAW | CO | AM | UZ | TG | SU | ST | ML | KK | NY | ZU | YO | TE | TA | SO| PA| NE | MN | MI | LA | LO | KM | KN
| JW | IG | HMN | HA | EO | CEB | BS | BN | UR | HT | KA | EU | AZ | HY | YI |MK | IS | BE | CY | GA | SW | SV | AF | FA | TR | TH | MT | HU | GL | ET | NL | DA | CS | FI | EL | HI | NO | PL | RO | CA | TL | IW | LV | ID | LT | SR | SQ | SL | UK
Copyright ©2015-2025 Shenzhen Slkor Micro Semicon Co., Ltd